Learning Outcomes
i. Delve into the captivating process of electrolytic refining, exploring how impurities are removed to produce pure copper.
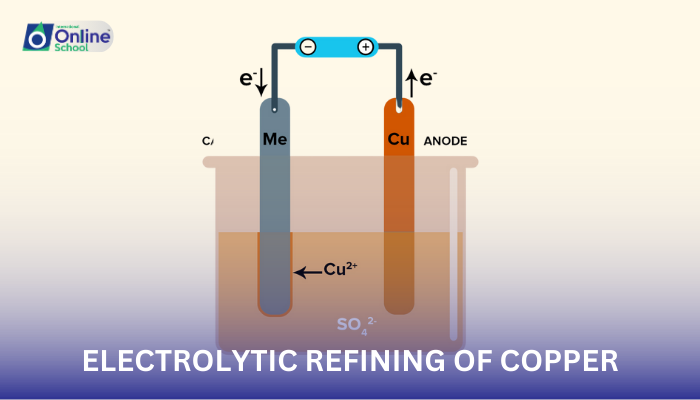
ii. Identify the key components of an electrolytic refining cell, including the anode, cathode, electrolyte, and separator.
iii. Understand the mechanism of electron flow and redox reactions that drive the purification of copper during electrolysis.
iv. Differentiate between primary and secondary copper, understanding the role of electrolytic refining in both cases.
v. Recognize the significance of pure copper in various industries and modern technologies.
Introduction
In the realm of chemistry, where elements undergo transformations, electrolytic refining emerges as an elegant process that purifies copper, transforming it from a crude material into a shining, versatile metal. Copper, with its remarkable electrical conductivity, thermal conductivity, and malleability, plays a pivotal role in various industries, from construction to electronics. This lesson will embark on a journey into the intricacies of electrolytic refining, illuminating the mechanism by which impurities are removed to produce pure copper.
i. The Electrolytic Refining Apparatus: A Symphony of Components
The electrolytic refining cell, the heart of the purification process, comprises four essential components:
Anode: The impure copper source, where oxidation occurs, releasing copper ions (Cu2+) into the electrolyte.
Cathode: A thin strip of pure copper, where reduction occurs, depositing pure copper atoms (Cu) from the electrolyte.
Electrolyte: A solution of copper sulfate (CuSO4) and sulfuric acid (H2SO4), providing a medium for ion movement and facilitating electron transfer.
Separator: A porous membrane that prevents direct contact between the anode and cathode, preventing short circuits while allowing ion flow.
ii. The Mechanism of Copper Purification: A Tale of Electron Flow and Redox Reactions
The purification of copper during electrolysis hinges on the principles of electron flow and redox reactions:
Anode: Copper atoms at the anode lose electrons, transforming into copper ions: Cu → Cu2+ + 2e-
Cathode: Copper ions from the electrolyte gain electrons, depositing as pure copper atoms on the cathode:
Cu2+ + 2e- → Cu
iii. Primary vs. Secondary Copper: Electrolytic Refining's Versatility
Electrolytic refining plays a crucial role in both primary and secondary copper production:
Primary copper: Pure copper is extracted from its ores through smelting and other processes. Electrolytic refining further purifies this copper, producing high-grade metal for various applications.
Secondary copper: Scrap copper, often from discarded electronics or machinery, is recycled through electrolytic refining, transforming it into pure copper once again.
iv. The Significance of Pure Copper: A Versatile Metal for a Modern World
Pure copper finds wide application in various industries and modern technologies:
Electronics: Copper is the backbone of electrical wiring, cables, and components due to its excellent conductivity.
Construction: Copper pipes and plumbing systems rely on copper's corrosion resistance and malleability.
Transportation: Copper plays a crucial role in automotive wiring, heat exchangers, and electrical components.
Electrolytic refining, a testament to human ingenuity, stands as an essential process that transforms impure copper into a pure, versatile metal. Understanding the mechanism of electron flow, the role of redox reactions, and the significance of electrolytic refining in both primary and secondary copper production empowers us to appreciate the profound impact of chemistry on our modern world.